Impurity Profiling and Characterization
In the vast realm of substance creation and analysis, impurities often emerge as the unsung villains. By definition, impurities are substances within a confined environment that are not the intended product. Their presence, particularly in pharmaceuticals, can be a cause for concern, necessitating the need for their thorough profiling.
Types of Impurities
Broadly speaking, impurities can be classed into several categories:
Organic Impurities: These often arise from the product itself or the processes it undergoes. For instance, degradation products of a drug molecule are organic impurities.
Inorganic Impurities: Metals, reagents, ligands – these often sneak into the final product from raw materials or the production apparatus.
Residual Solvents: Often overlooked, solvents can sometimes linger longer than they should, raising safety concerns.
Others: Sometimes, it’s the microscopic invaders, like microbes, or tangible ones, like particulate matter, that cause the alarm.
Sources of Impurities
While the aforementioned classifications sound tidy, their origins are manifold:
Raw materials may bring along unintended guests.
The manufacturing process might inadvertently produce or introduce contaminants.
Improper storage conditions can degrade products.
Even the packaging, seemingly benign, can be a source!
Regulatory Guidelines on Impurity Profiling
Dive into the guidelines set by International Council for Harmonisation (ICH) – Q3A, Q3B, and Q3C, and you'll find an intricate web of regulations weaving through national and international directives. These criteria don't just lay out acceptable thresholds; they ensure patient safety and product efficacy.
Techniques for Impurity Characterization
The arena of impurity analysis is replete with cutting-edge techniques. Spectroscopic methods like NMR or MS give us a detailed molecular snapshot. Chromatographic techniques like HPLC or GC segregate impurities for precise identification. And tools like ICP-MS or AAS probe the elemental compositions.
Qualitative and Quantitative Analysis
An impurity's presence is one thing; quantifying it is another ball game. Method development is both an art and a science, demanding sensitivity, selectivity, and specificity. But once validated, these methods can detect even the most elusive of impurities.
Impurity Profiling in Drug Development
Imagine the journey of drug development as a winding road. Impurities can pop up at the initial stages, lurk during the scale-up, or surprise us during production. Their potential impact on a drug's efficacy, safety, or stability makes their profiling indispensable.
Challenges in Impurity Profiling
Ah, the challenges! Some impurities remain enigmatic. Others, present in minuscule amounts, test the limits of detection. And then there are those so close in nature that differentiating them becomes a Herculean task.
Case Studies
History is rife with instances where impurities played unexpected roles. Some sparked groundbreaking research, while others led to major recalls. These tales are not just cautionary but also enlightening, showcasing the relentless pursuit of safety.
Peek into the present scenario of impurity profiling, and it’s clear: it's an ever-evolving field. The increasing demands for product quality and safety underscore its significance. The journey towards purity, though challenging, is unarguably crucial.
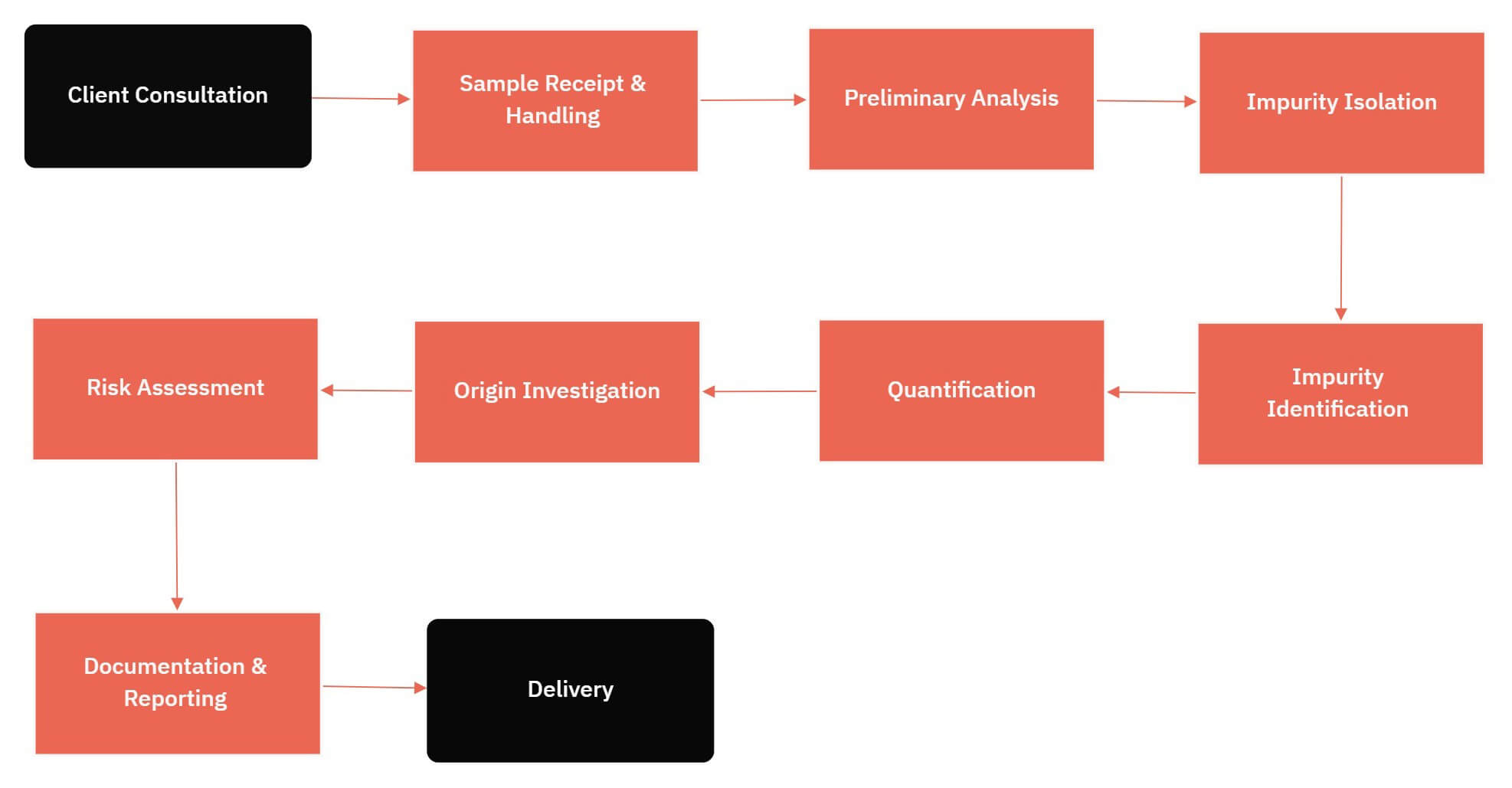
Definition & Importance of Impurity Identification
It's the process of determining the nature of unknown materials in products using various analytical techniques. Impurities in any product can delay development or lead to product recalls. Chemical impurity analysis is crucial in medical device and pharmaceutical development as it affects the safety and efficacy of the final therapeutic products.
Sources of Impurities
Impurities can arise from the manufacturing process and/or storage. They include raw materials, by-products, intermediates, degradants, reagents, catalysts, heavy metals, and other materials.
Types of Impurities & Regulations
The three main types evaluated are organic impurities, inorganic impurities, and residual solvents. Organizations like the FDA and ICH offer guidelines on testing and acceptable limits of impurities in medical or pharmaceutical products.
Smolecule Lab's Impurity Identification Services
Provides impurity identification and characterization for various industries.
Equipped with the analytical instrumentation and expertise for isolating, analyzing, and identifying impurities.
Compliance with FDA and ICH guidelines for impurities.
Can prepare and characterize reference standards upon identifying impurities. Also, capable of developing and validating methods to monitor these impurities.
Stability Programs & Impurity Formation: Chemical impurities can develop when products are stored for extended periods. Smolecule Labs can simulate various conditions to develop an impurity profile through forced degradation during stability programs.
Problem-Solving Services
Smolecule Labs offers services to determine the source of impurities, like analyzing raw materials or delving into the manufacturing process. They provide tailored solutions for impurity identification and analysis needs.