G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. GPCRs utilize trimeric G proteins to transduce information from the extracellular environment and other cells to intracellular signals that modulate cellular responses. They are not only the largest and most diverse family of cell membrane receptors, but are, in fact, one of the largest protein classes in mammals. With more than 800 genes in this receptor family, and binding to a vast array of ligands, GPCRs play a role in multiple physiological functions including sight, taste, smell, neurotransmission, pain perception, and immune responses.
The 2012 Nobel Prize in Chemistry was awarded to Brian Kobilka and Robert Lefkowitz for their work that was "crucial for understanding how G protein-coupled receptors function". There have been at least seven other Nobel Prizes awarded for some aspect of G protein–mediated signaling. As of 2012, two of the top ten global best-selling drugs (Advair Diskus and Abilify) act by targeting G protein-coupled receptors.
GPCRs are categorized into six classes based on sequence and function, namely Class A—rhodopsin-like receptors, Class B—secretin family, Class C—metabotropic glutamate receptors, Class D—fungal mating pheromone receptors, Class E—cAMP receptors, and Class F—frizzled (FZD) and smoothened (SMO) receptors. All GPCR members share a common seven transmembrane (7TM) architecture linked by three extracellular (ECL) and three intracellular (ICL) loops. However, they have low sequence identity and possess different extracellular N-terminal domains and diverse ligand-binding pockets
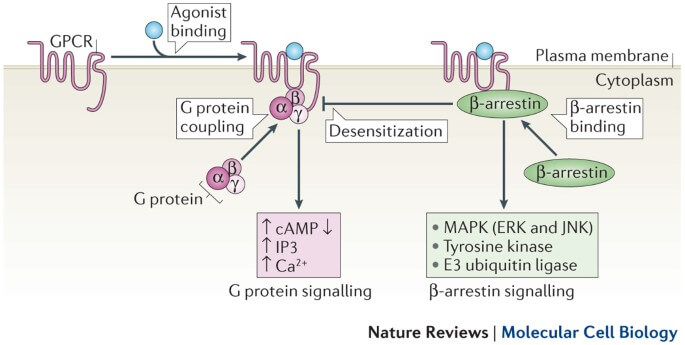
GPCR signaling is initiated when a ligand binds to the extracellular surface of the GPCR. This results in a conformational change in the GPCR causing the activation of the Gα subunit. The activated Gα exchanges bound GDP with GTP, resulting in the disassociation of the Gα subunit from the Gβγ dimer. The Gα and Gβγ subunits then induce or inhibit intracellular signaling cascades as a response to the extracellular stimuli. Ligand dissociation from the GPCR allows for binding of a new inactive heterotrimeric G protein complex and subsequently another round of signaling.
GPCRs are considered the largest family of targets for approved drugs. Roughly a third of all drugs approved by the U.S. Food and Drug Administration target GPCRs. Numerous factors contribute to the wide utility of GPCR-targeted drugs, including their druggability, interaction with numerous types of chemical entities, and expression in the plasma membrane, which facilitates molecular interactions in the extracellular milieu. Enormous efforts have been expended to find relevant and potent GPCR ligands as lead compounds. Non-olfactory GPCRs constitute more than half of the human genome encoded targets that are not yet exploited for any therapeutic use and the knowledge is disproportionately focused in the scientific literature. Preliminary studies highlight that these receptors have functions in genetic and immune system disorders. While the drugs that currently target GPCRs are primarily small molecules and peptides, GPCRs also recognize diverse ligands, including inorganic ions, amino acids, proteins, steroids, lipids, nucleosides, nucleotides, and small molecules
But like many therapeutics, drugs that target GPCRs often have side effects, some of which can be serious. For example, drugs that target a group of GPCRs called opioid receptors are very effective at treating pain but also have dangerous side effects such as respiratory distress and constipation. At the moment, these compounds are unable to target the pain-alleviating signaling pathway without also activating the respiratory and gut pathways.
Now, an interdisciplinary team of researchers has gained new insight into the way GPCRs operate, a step toward the development of improved drugs with fewer side effects. “Drugs that target GPCRs are used to treat a wide variety of disorders in medicine—heart disease, lung disease, sleep and neuropsychiatric disorders—and GPCRs are responsible for smell, taste, and vision as well,” says senior author Jonathan A. Javitch, MD, PhD, the Lieber Professor of Experimental Therapeutics in Psychiatry at Columbia University Vagelos College of Physicians and Surgeons and chief of molecular therapeutics at the New York State Psychiatric Institute.
The nonvisual arrestins, β-arrestin (βarr) 1 and 2 (also known as arrestin 2 and 3, respectively), are recruited by hundreds of different GPCRs to desensitize G protein signaling and to promote clathrin-mediated receptor endocytosis. By scaffolding kinases and other molecules, receptor-activated β-arrestins can also directly facilitate diverse cellular signaling pathways distinct from those initiated by G proteins. There is great interest in identifying therapeutics that preferentially promote or block βarr signaling to allow for more efficacious and specific targeting with the potential of reduced side effects. Structural and kinetic insights into the mechanism by which ligand-mediated GPCR activation enables βarr coupling and activation are required to achieve this goal.
“In our study, we use methodology that allows us to probe in unprecedented detail how drug-stimulated GPCRs activate β-arrestin, a protein involved both in terminating some signals and mediating others,” says Wesley B. Asher, PhD, co-first author and assistant professor of clinical neurobiology in the Department of Psychiatry at Columbia, “with the ultimate goal of enabling the development of pathway-specific compounds.”
The researchers use total internal reflection fluorescence (TIRF)-based single-molecule fluorescence resonance energy transfer (smFRET) imaging to investigate the β-arrestin1 (βarr1) activation mechanism by examining release of its C-terminal tail region (βarr1 tail) upon binding of phosphorylated receptor or known mimics of the Rp tail. These measurements enabled direct observation of stochastic, dynamic processes in the βarr1 tail—largely inaccessible to ensemble methods—associated with βarr1’s interaction with the receptor and of how these processes are regulated by GPCR activation.
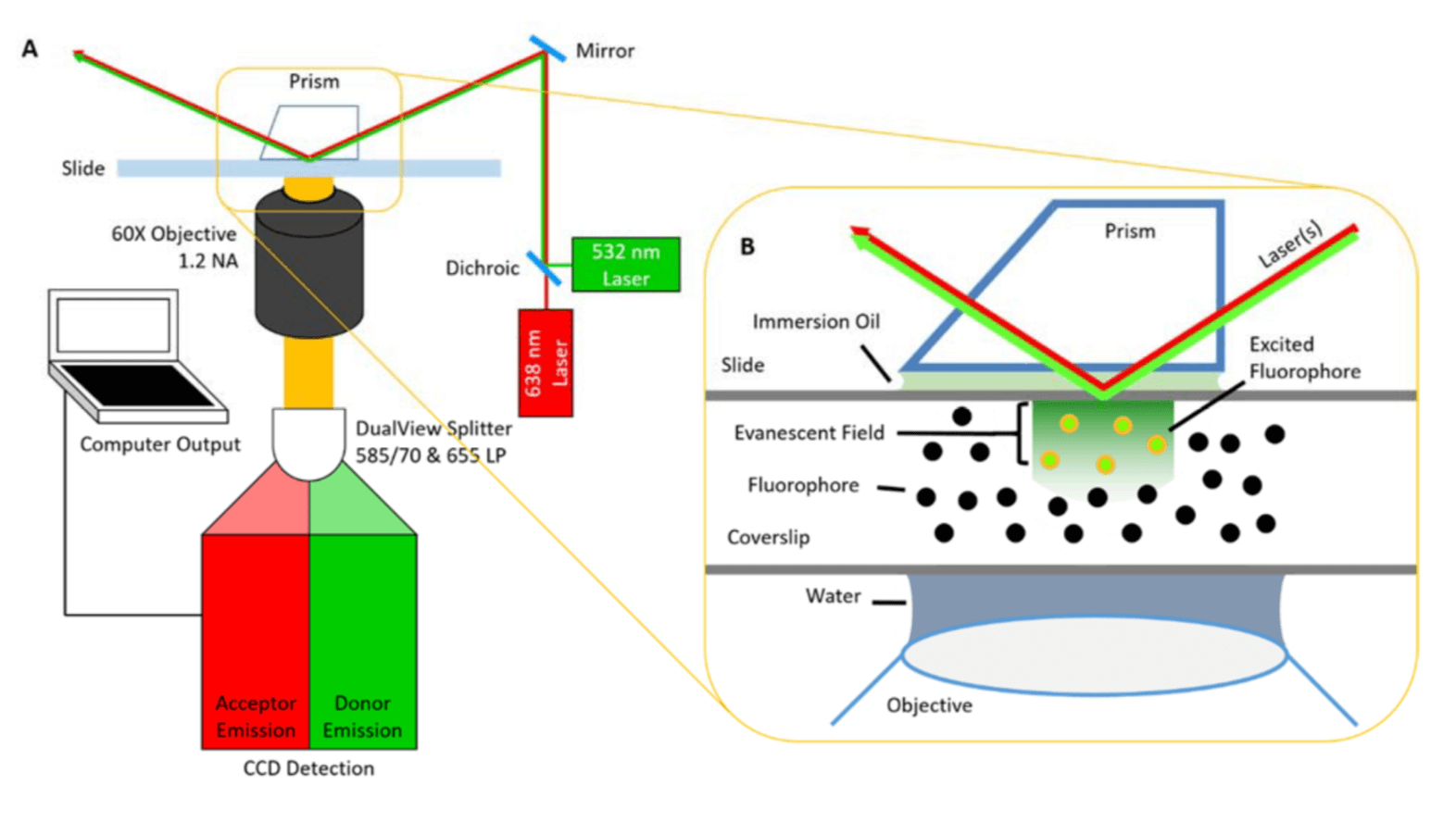
With smFRET, the team decided to probe the beta-adrenergic receptor—a prototypical GPCR broadly relevant to many different areas of biology. Binding of drugs or endogenous hormones to beta-adrenergic receptors or other GCPRs on the cell’s exterior membrane leads to signals on the inside of the cell that are mediated by activation of G proteins. But binding of another type of protein, β-arrestins, terminates this signaling and can activate other—desired or undesired—downstream pathways.
The team leveraged their insights from smFRET imaging for targeted, hypothesis-driven studies in living cells to show that agonist-stimulated, full-length, wild-type β2-adrenergic receptor (β2AR) is unable to recruit β-arrestin2 (βarr2) in the absence of GRK expression, whereas truncation of the receptor’s distal C tail reveals measurable GRK-independent βarr2 recruitment. These findings provide evidence for receptor autoinhibition mechanisms that contribute to the regulation of agonist-induced βarr engagement, downstream signaling, and receptor internalization.